by Dr Jay Lu
In this mini blog, we would like to highlight one of the most exciting applications in optical mapping: Cardiac Regeneration. Due to a shortage of organ donors and the limited retention of cellular therapies, cardiac engineering has created a process to generate functional cardiac tissues. Cardiac regeneration provides an alternative to the reparation of damaged heart tissue and restores cardiac function (1,2). One of which, cardiac patches appear to hold a huge potential to repair damaged heart tissue and restore cardiac function.
Background
Cardiovascular disease is often associated with myocardial infarction (MI), a prominent factor of morbidity and mortality worldwide (3). The heart is composed of dynamic and multicellular tissues that exhibit specific structural and functional characteristics. The cardiac muscle in an adult lacks the capabilities of self-repair and regeneration after MI. After the anguish of irreversible cardiac muscle death, the cardiomyocyte (CM) will gradually become replaced by fibrotic scar tissue (4). Fibrotic scar tissue is different from the rest of the heart as it does not contract or pump and does not contain new heart muscle cells. Therefore, the loss of contractile capacity leads to the dysfunction of the heart and eventually causes heart failure (5). Thus, why we still face challenges in exploring novel therapeutic methods for myocardium regeneration.
Within the past decade, there have been tremendous advances in biomaterial and tissue engineering. The bioengineered cardiac patch shows promising potential for the restoration of cardiac function (6). The cardiac patch provides seeded cells with additional thickness and rigidity. It works by attaching to the epicardial surface of the myocardium to optimize cell retention and engraftment. Hence, providing transplanted cells with a microenvironment for new tissue growth.
Human pluripotent stem cells provide important cell sources for cardiac patches due to their self-renewal capacity and ability to generate any cell type in the human body. These cells develop paracrine factors for heart repair, including growth, antifibrotic, and antiapoptotic (7). Emerging preclinical studies have been reported effective in improving myocardial remodeling (8-11).
How can Optical Mapping improve Cardiac Regeneration?
In our previous blog post, we discussed how arrhythmia is one of the main areas for optical mapping and how it can decipher complex patterns of cardiac excitation propagation. Arrhythmias are strongly related to MI, which results from abnormal conduction through the area of infarct with re-entrant circuits or from direct interruption of the conduction system.
Optical mapping grasps high-speed, fluorescent-based imaging to measure electrical properties such as membrane potential (Vm) and intracellular calcium transients (CaT). Therefore, providing multicellular cardiac formulations at high spatiotemporal resolution. The ability to map all phases of electrical activity on cardiac patched epicardial surfaces allows researchers to determine the therapeutic efficacy of the cardiac patch with more physiological sophistication and clinical relevance.
A good example:
Below is a recent dataset of the cardiac patch experiments imaged by an optical mapping system. The membrane potential in the MI group shows rough, discontinued conduction while compared to Sham (the control). However, the cardiac patch in the treated group shows smooth membrane potential by initiation and conduction, suggesting the cardiac function has become restored by the cardiac patch treatment.
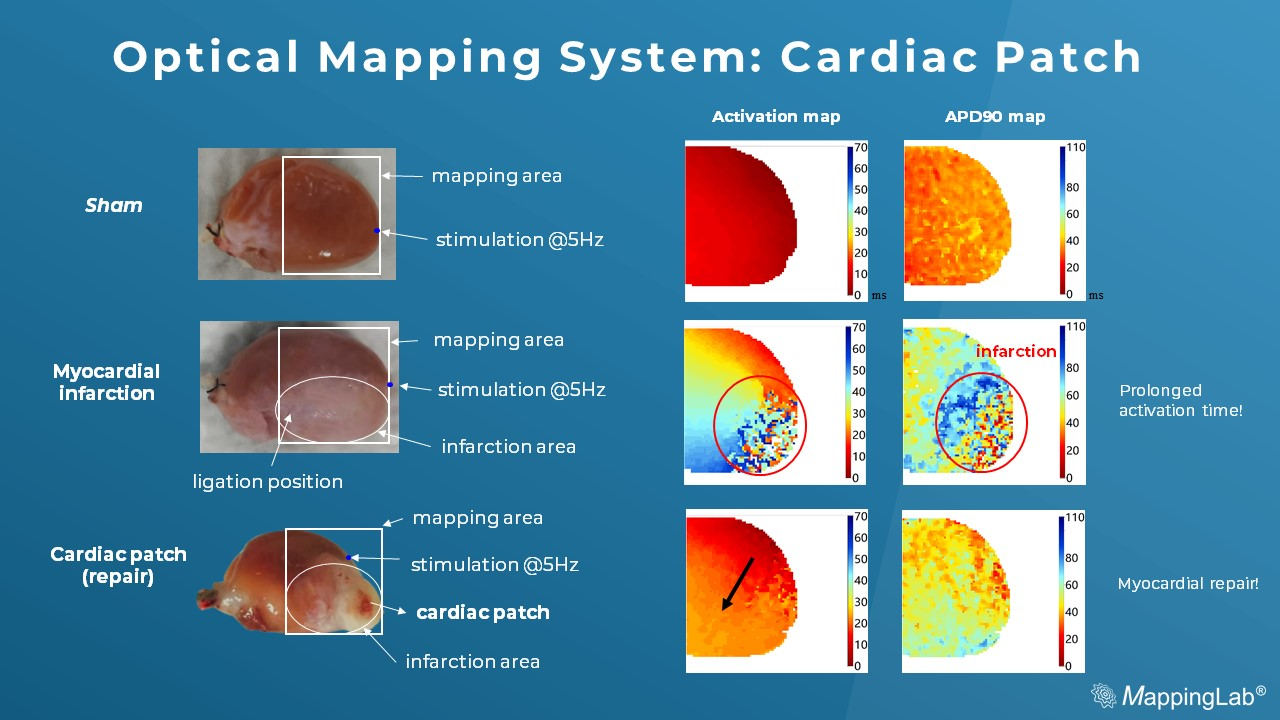
Preliminary data: courtesy of Kaifeng Key Laboratory of Cardiac Electrophysiology, Henan, China
Reference
- Weinberger, Florian et al. “Engineering Cardiac Muscle Tissue: A Maturating Field of Research.” Circulation research vol. 120,9 (2017): 1487-1500. doi:10.1161/CIRCRESAHA.117.310738
- Ogle, Brenda M et al. “Distilling complexity to advance cardiac tissue engineering.” Science translational medicine vol. 8,342 (2016): 342ps13. doi:10.1126/scitranslmed.aad2304
- Laflamme, Michael A, and Charles E Murry. “Heart regeneration.” Nature vol. 473,7347 (2011): 326-35. doi:10.1038/nature10147
- Mattera, Rosanna et al. “Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes.” Nutrients vol. 9,5 523. 20 May. 2017, doi:10.3390/nu9050523
- Robison, Patrick et al. “Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes.” Science (New York, N.Y.) vol. 352,6284 (2016): aaf0659. doi:10.1126/science.aaf0659
- Mei, Xuan, and Ke Cheng. “Recent Development in Therapeutic Cardiac Patches.” Frontiers in cardiovascular medicine vol. 7 610364. 27 Nov. 2020, doi:10.3389/fcvm.2020.610364
- Moretti, Alessandra et al. “Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification.” Cell vol. 127,6 (2006): 1151-65. doi:10.1016/j.cell.2006.10.029
- Gao, Ling et al. “Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine.” Circulation vol. 137,16 (2018): 1712-1730. doi:10.1161/CIRCULATIONAHA.117.030785
- Gaetani, Roberto et al. “Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction.” Biomaterials vol. 61 (2015): 339-48. doi:10.1016/j.biomaterials.2015.05.005
- Huang, Ke et al. “An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs.” Science translational medicine vol. 12,538 (2020): eaat9683. doi:10.1126/scitranslmed.aat9683
- Yeung, Enoch et al. “Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo.” Journal of tissue engineering and regenerative medicine vol. 13,11 (2019): 2031-2039. doi:10.1002/term.2954

