By Dr Jay Lu
Coronavirus, COVID-19, or simply as the infamous pandemic that plagued the world since late 2019, we have all heard about the virus spreading across the globe. But how many of us understand the real statistics and risks behind COVID 19? In this article, we aim to shed some light on the virus, potential treatments, and the possible risks involved
The Deadly Path Of COVID-19
Since its unforeseen birth in December 2019 in the Wuhan province of China, the COVID-19 disease (caused by Severe Acute Respiratory Syndrome Coronavirus 2; SARS-CoV-2) has become responsible for over 97,500 deaths worldwide (at the time of writing). This deadly virus is categorized as a respiratory illness with common symptoms including fever, cough and, in more extreme cases, shortness of breath. Scientists have recommended an incubation period of between 5 and 14 days from initial exposure to the virus to the development of symptoms.
With deaths rising, and an anticipated peak on the horizon, it has never been so crucial that medical professionals seek a safe and efficient therapy to impede the spread of COVID-19 infections. While there are no FDA-approved drugs to combat COVID-19, there has been much investigation and debate into either novel and re-purposed agent, such as remdesivir or a blend of hydroxychloroquine (HCQ) and azithromycin, as possible COVID-19 therapies (1). Each drug is currently being evaluated in clinical trials for their abilities to inhibit the growth of SARS-CoV-2.
Current Possible Treatments
While there is more research and hypothesis over solid and definitive evidence for an appropriate treatment drug, medical professionals worldwide are working together to deduce the best immediate drugs for “off-label” or “compassionate use” treatments. The two most common are lopinavir/ritonavir, and HCQ – which is famous for its antimalarial properties (1).
It has been found that HCQ treatment is mainly connected with viral load reduction in sufferers of COVID-19; its effect is bolstered by its ability to treat bacterial infections. In one hand, HCQ is becoming a firm favorite in the race for a cure, earning the acknowledgment of the US President as a ‘game-changer’ drug. However, research has been conducted on a relatively small scale with limited evidence of its long-term impacts, possible side effects, and availability. As such, the study comes with its limitations.
The Risks of Arrhythmia and Sudden Cardiac Death?
In 2017, the WHO published a review on pharmacovigilance, clinical and preclinical data surrounding anti-malarial drugs, including HCQ (2). The study divulged the association between these drugs and QT interval prolongation, which is when the heart muscle takes longer to recharge between beats. This process is most notably hindered when there is a block of the human ether-à-go-go related gene (hERG) potassium (K+) channel. Blockade of the hERG channel lengthens ventricular repolarization and the duration of ventricular action potential. This is reflected on the surface ECG as a prolonged QT interval; it may also result in the reactivation of inward, mainly calcium, depolarizing currents, thereby generating early afterdepolarizations (EAD). It is worth noting that examining hERG block and QT prolongation are the essential determinants in the Comprehensive in Vitro Proarrhythmia Assay (CiPA), part of FDA risk assessment.
Recently, the American College of Cardiology (ACC) and Mayo Clinic Proceedings independently put together urgent guidelines surrounding the use of HCQ-Azithromycin (3,4). In their articles, they express the concern whereby HCQ-Azithromycin may cause unwanted the heart rate corrected QT interval (QTc), thereby increasing the risk of drug-induced Torsades de pointes (TdP). TdP is a potentially lethal polymorphic ventricular tachycardia which can cause drug induced-sudden cardiac death (SCD) from individual or concurrent use of these medications (5). Although ACC concluded the overall benefit was greater than the cardiovascular risk in COVID-19 patients, the intensity of QT and arrhythmia monitoring should be carefully considered in the context of risk level, resource availability, and quarantine considerations.
For those who are interested in catching up with some COVID-19 background and latest development, please visit our previous blog article and FDA website https://bit.ly/2Rj06V5 for more information.
Technology that Can Help?
Fortunately, yes! This is where the news becomes a little more optimistic. Cardiac mapping technology has been built to solve this exact problem way before newly identified drugs or re-purposing drugs entering clinical trials. A recent report of clinical trial in Brazil, the higher dosage of chloroquine has been linked to a higher cardiovascular risk and little effect on viral clearance (6). Just a few days ago, a research group in China potentially proved that chloroquine diphosphate indeed had dose-dependent effects on Langendorff hearts from Guinea pigs. The electrical mapping system from MappingLab (see video) was employed to simultaneously record field potentials from the left atria and ventricle. Not only was the significant delay shown in activation conduction map (Panel A) and repolarisation trace (Panel B) from the left ventricle, but the severe drop on heart rate at a higher dose (100 µM) was also clearly demonstrated (Panel C).
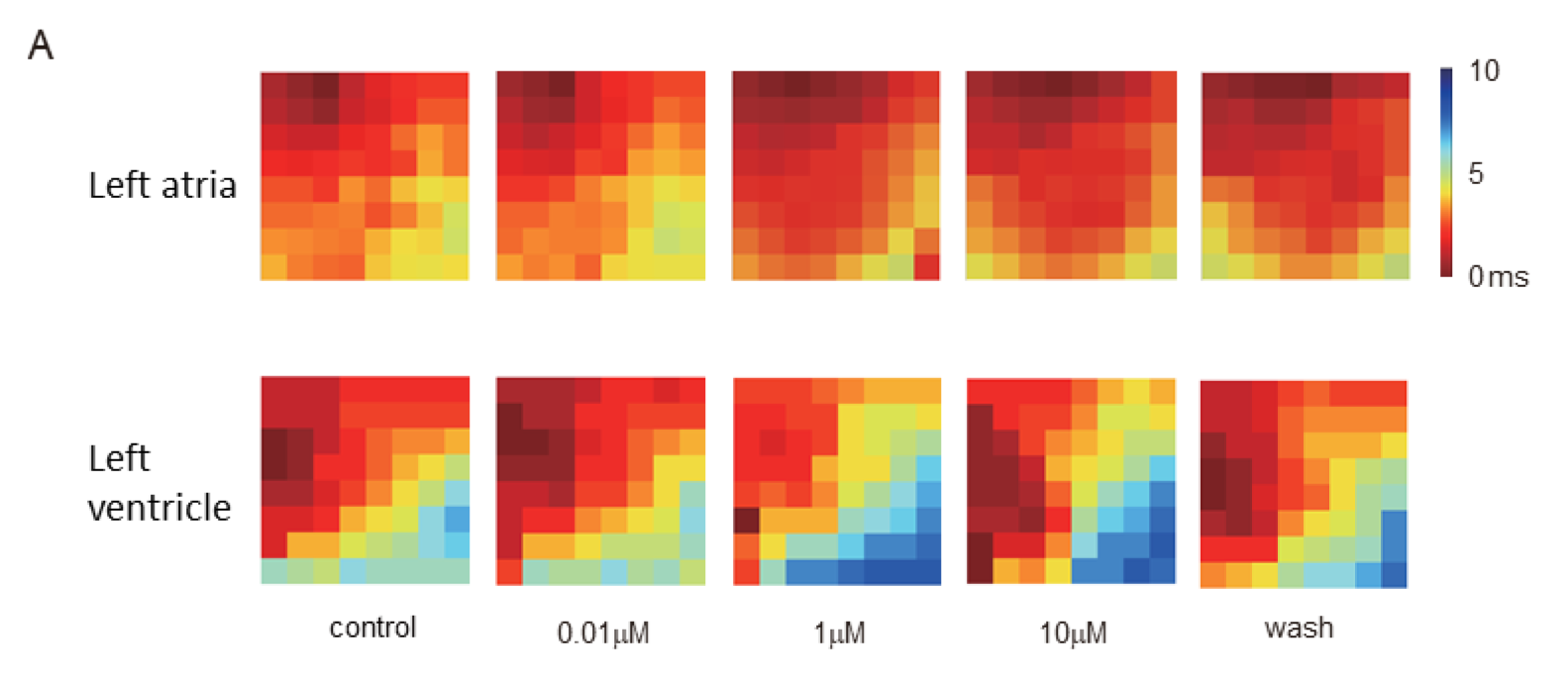
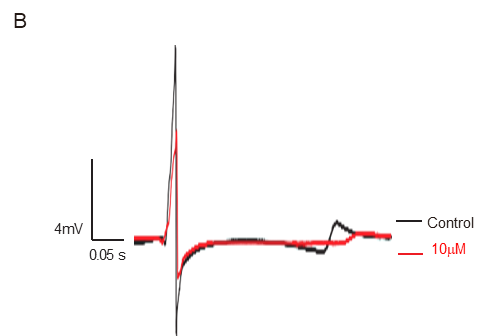
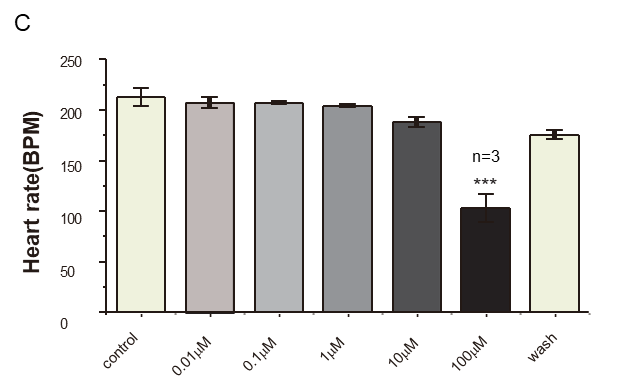
Preliminary data: courtesy of University of Oxford and Kaifeng Key Laboratory of Cardiac Electrophysiology, Henan, China
With help of our powerful mapping systems, scientists can easily pinpoint the exact areas of the heart that are diseased by identifying all important electrophysiological parameters with precision and reliability.
The results are easily replicated; further electrophysiological insights contribute to more refined understanding and awareness of abnormalities in various ion channels in the heart. As further clinical tests are conducted and greater data is gathered, mapping technology will inevitably pose as a key player in the plight for a COVID-19 treatment. In other words, our technology not only can expedite the drug development process against SARS-CoV-2, but also greatly increase the possibility of clinical trial success and subsequent FDA approval.
Final Words
As medical professionals work around the clock to discover a proficient therapy to counteract coronavirus, there is plenty that people can do in the meantime to hinder its spread. We are constantly advised to wash our hands and maintain a 2m distance from others. We are advised to only go shopping if it is essential. While advancement towards the discovery of a suitable vaccine is gathering steam at an astronomical level, it is predicted that we are still at least 12 months away. The conclusion of the virus will be a fine balance between an economic recession, grief, but also a budding hope in the form of the medical industry.
Lastly, the assessment of QT/QTc interval prolongation is an important part of the regulatory evaluation of new medicines and has become a common reason for drugs being withdrawn from the market. Our mapping technology provides a rudimental tool for screening drug cardiotoxicity by looking into all important cardiac indices and seeing the drug that has any adverse effect on cardiac electrophysiology. We are confident that our technology will enable many pharmaceutical companies to waltz through the CiPA and FDA-approved drug development process. This will undoubtedly help millions of people in a difficult situation like this pandemic.
Reference
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2.pdf
- https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19
- https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/jmcp/jmcp_covid19-1585757281070.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6192813/
- https://www.medrxiv.org/content/10.1101/2020.04.07.20056424v2.article-metrics