by Dr Jay Lu
What is CiPA?
The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative is created collectively by an international multidisciplinary team of regulatory, industry, and academic scientists to develop a new paradigm for the cardiac safety evaluation of new drugs that provides a more accurate and comprehensive mechanistic-based assessment of proarrhythmic potential.
It is designed to focus on assessing the proarrhythmic risk of drugs, building on the emergence of new technologies, and expanding understanding of torsadogenic mechanisms beyond the hERG block.
Main pillars of the CiPA
According to the latest meeting report from a Cardiac Safety Research Consortium, Health and Environmental Science Institute, and FDA meeting, there are 4 components of the CiPA paradigm¹ :
- Drug effect on multiple human cardiac currents
- In Silico modeling to generate a Torsade Metric Score
- Translational assessment using human-induced pluripotent stem cell derived cardiomyocytes
- Clinical ECG assessments including QT prolongation and QT morphological changes
Cardiac mapping systems join forces…
To gain a more comprehensive and holistic view of how drugs may affect the whole heart, cardiac mapping technology is undoubtedly a necessary tool in exploring these, on top of the CiPA initiative, and serves as an excellent complement to the third pillar of CiPA, where the use of human iPSC-CMs is inevitably lacking nativeness and maturity.
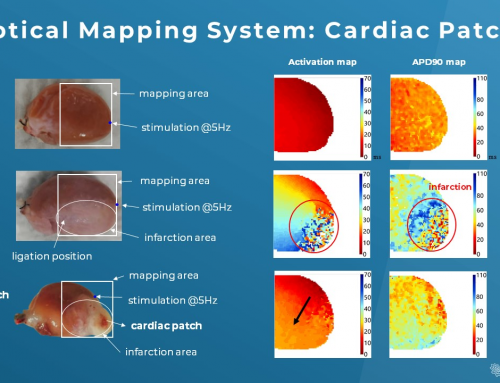
Cardiac mapping technology can help scientists easily pinpoint the exact areas of the heart that are diseased by identifying all important electrophysiological parameters and then displaying that information similar to a geographic map (known as an isochronal conduction map). The best thing is these experimental results are highly (and easily!) replicable.
Let’s dive into a quick example…
A recent study published by Wang et. al. ² suggested the adverse effects of hydroxychloroquine (HCQ), which could be amplified with the addition of azithromycin (AZM) in a Langendorff Guinea pig heart. In addition to the current CiPA core ion channel investigation with a patch clamp technique, they utilized cardiac electrical mapping technology to discover the slower spontaneous heartbeats and ventricular conduction velocity, delayed repolarization, and discordant conduction with the treatment of both drugs.
Subsequently, they further explored the mechanism of these abnormal cardiac electrical phenomena in the presence of HCQ & AZM with the help of the optical mapping technology. They found that the prolongation of action potential duration (APD) and aberrant intracellular Ca2+ transient resulted in slower conduction and ventricular tachycardia.
Their data looks very promising and it is safe to say that the electrical and optical mapping systems can have tremendous benefits in massive electrophysiological data acquisition from ex vivo and in vivo cardiac samples. They can be operated independently or in conjunction to maximize the full potential of cardiac mapping technology.
Final words
The implementation of the CiPA initiative has been successful in preventing the introduction of drugs with torsadogenic risks to the market. However, the high sensitivity of this methodology may cause low specificity. As a result, many drugs with effects on hERG blockade and QTc prolongation – but little actual torsadogenic risk – have been deprioritized or excluded from further development (false positive). Alternatively, if approved, they may see their clinical use limited by inappropriate warnings in product labeling. Not all drugs that block hERG are correlated with torsadogenic risk, and some drugs with QTc prolongation do not necessarily inhibit hERG acutely ³.
This is where cardiac mapping comes in and serves as a great COMPLEMENT to the current CiPA approach; it provides researchers a more comprehensive methodology of detecting cardiac electrophysiological properties straight from cardiac tissue and/or the whole heart. By introducing cardiac mapping systems to the current CiPA approach, cardiac safety screening will unquestionably become more precise and wide-ranging. This will save pharmaceutical companies millions of dollars in pre-clinical development and subsequent clinical trials.
Reference
- https://link.springer.com/article/10.1177/2168479018795117
- https://www.biorxiv.org/content/10.1101/2020.05.21.108605v3
- https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1755-5922.2010.00154.x